Optimising bacteriophage amplification as seen in Capsid and Tail
Introduction
The issue of low PFU/ml results when amplifying phages is a common problem facing researchers worldwide. With a focus on quality over quantity at the early development stage, this is often found to be an issue when scaling up for product trials then full scale manufacturing. Increasing PFU/ml at this stage provides more phage product for use in trials or for selling onto clients.
Using flasks for phage amplification
At research level, amplification of phages in laboratories is commonly carried out in Erlenmeyer flasks, with air being pumped into the media using a pipette as shown in the diagram. The flask is then transferred to an incubator for temperature control. While this technique produces phage product, there are inherent drawbacks to the method which contribute to low and inconsistent PFU/ml results.
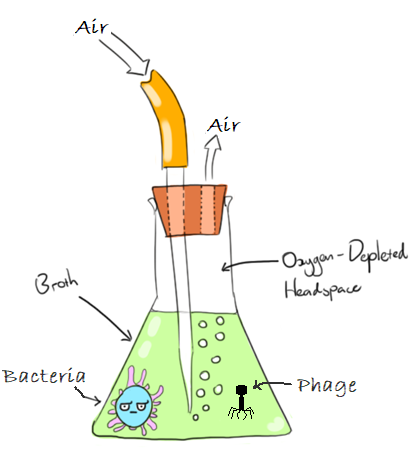
Flask bioreactor process
Difficulties of working with flasks
- Lack of control due to empiric nature of the flask.
- Inability to control temperature effectively in the incubator.
- Inability to control gas flow at optimum rate.
- Risk of contamination.
- Labour intensive.
- Significant downtime between each experiment with cleaning and sterilisation required.
- Difficult to scale up.
Revolutionary Technology
Working with client trials, we discovered that the following parameters are crucial in optimising phage amplification:
- Temperature control
- Gas flow rate
- Contamination reduction
Having identified these issues and key parameters, we have developed a new and revolutionary solution to improve phage manufacturing. The CellMaker uses a patented, revolutionary airlift technology which provides optimal aeration, and our single-use technology reduces downtime and eliminates the need for cleaning.

The CellMaker bioreactor
The CellMaker has several key benefits to optimise phage amplification:
- Accurate temperature control to +/-0.1°C provided by a Peltier plate situated within the Enclosure unit.
- Mass flow controllers allow for accurate gas flow rate.
- Sterile bioreactors fitted with tubing and connections for “plug and play” convenience reduces contamination.
- Single-use technology reduces downtime by eliminating the need for cleaning and sterilisation.
- Flexible solution with working volumes from 3L to 50L.
- 21 CFR part 11 compliant software.
Results from the CellMaker
Our technology is flexible and can be used across industries. The CellMaker is currently in use in the animal health, food safety, crop protection and drug discovery industries. Our technology has successfully amplified phages from E. coli, Salmonella, Agrobacterium, Listeria, Pseudomonas aeruginosa, Pseudomonas syringae, Staphylococcus aureus and Yersinia ruckeri all producing exciting results.
A selection of results are shown below:
| Bacteria | Inoculum PFU/ml | Final PFU/ml | Amplification Factor |
| Salmonella | 2 x 105 | 1 x 1010 | x 50000 |
| Agrobacterium | 5 x 105 | 1.25 x 1010 | x 25000 |
| Escherichia coli | 1 x 106 | 3 x 1010 | x 30000 |
| Pseudomonas syringae | 4 x 106 | 3 x 1010 | x 30000 |
Reduced manufacturing costs
Manufacturing costs is key to the success or failure of a product, and the significance of choosing the best technology for scaling up your process cannot be underestimated.
The CellMaker is producing excellent results within phage amplification due to the ability to control process parameters, the speed and ease of use of single-use technology, and the efficiency of the airlift process. This results in a faster route to market and optimised titers.
Read more about our results in our case studies.
This article was first published on the Phage Directory’s Capsid and Tail.