Rise of the Superbugs
Antimicrobial resistance (AMR) is predicted to become one of the biggest threats to public health globally
Antimicrobials are medicines used to treat infectious disease in humans, animals and plants. Their importance in the modern world cannot be overstated, however years of overuse and misuse has led to infectious agents such as bacteria, viruses and fungi developing mechanisms to survive exposure to our most commonly used drugs. These resistant microbes, or superbugs are responsible for increasing numbers of deaths worldwide with antibiotic resistance being of particular concern. According to a 2024 publication using statistical modelling, it is estimated that 4.71 million deaths in 2021 were associated with bacterial AMR, of these, 1.14 million were directly attributed to it (1). As bacterial AMR continues to grow, traditional antibiotics are becoming less effective, leaving doctors with fewer treatment options. It has now become crucial to explore alternative options to antibiotics, one such solution is the use of bacteriophages.
Tackling antimicrobial resistance: the potential of bacteriophages as an alternative treatment
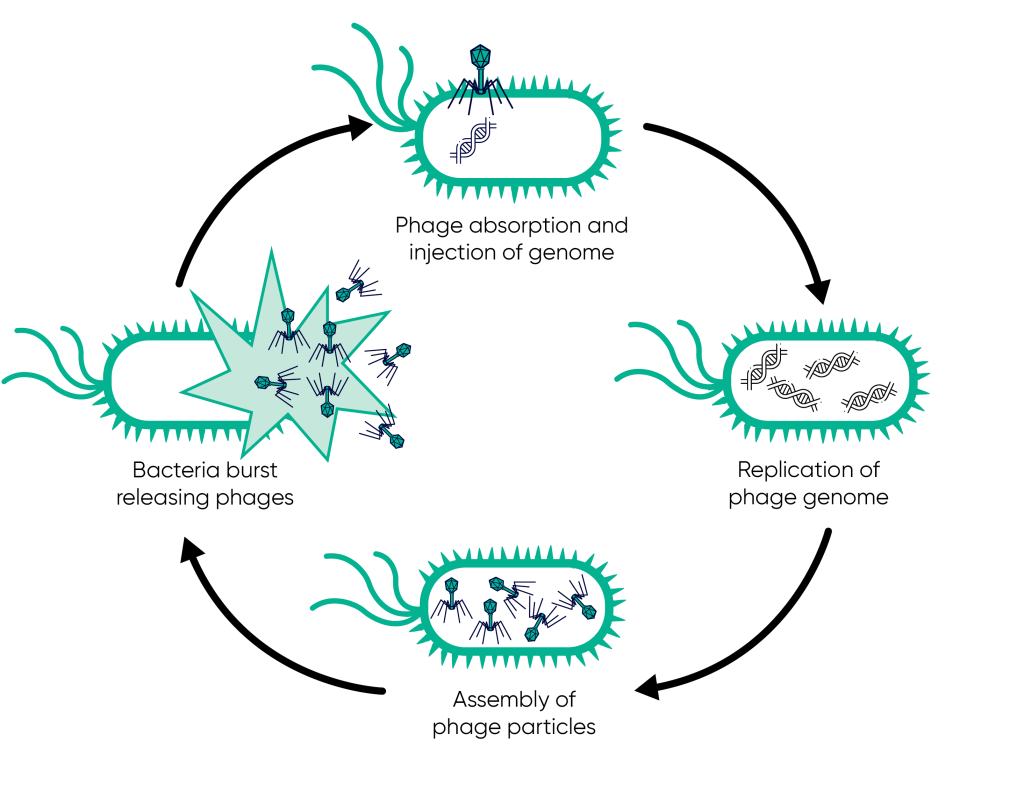
Bacteriophages, or phages, are viruses that specifically infect bacteria. On binding their target bacterium they inject genetic material, hijacking the host cell machinery to replicate the phage genome and assemble new virus particles. The bacterial host manufactures new phage particles until lysis is triggered, releasing new phage which will repeat this bacteriolytic replication cycle. Bacteriophages are incredibly diverse and can be found in virtually every environment where bacteria exist, from soil to seawater. Unlike antibiotics, which are chemically synthesised and designed target bacterial structures shared by many species, phages are highly specific, meaning they only infect particular strains of bacteria. This specificity means that while antibiotic treatment can lead to destruction of both harmful and helpful bacteria in the body, bacteriophages can be tailored to target only the harmful pathogens responsible for infection. Moreover, bacteriophages can evolve alongside bacteria. Since phages are constantly in a co-evolutionary battle with bacteria, they have the ability to adapt to and overcome bacterial resistance mechanisms. This makes them a dynamic and potentially long-term solution to the AMR crisis, in contrast to antibiotics which can become ineffective over time as resistance develops.
Phage therapy is not a one-size-fits-all solution
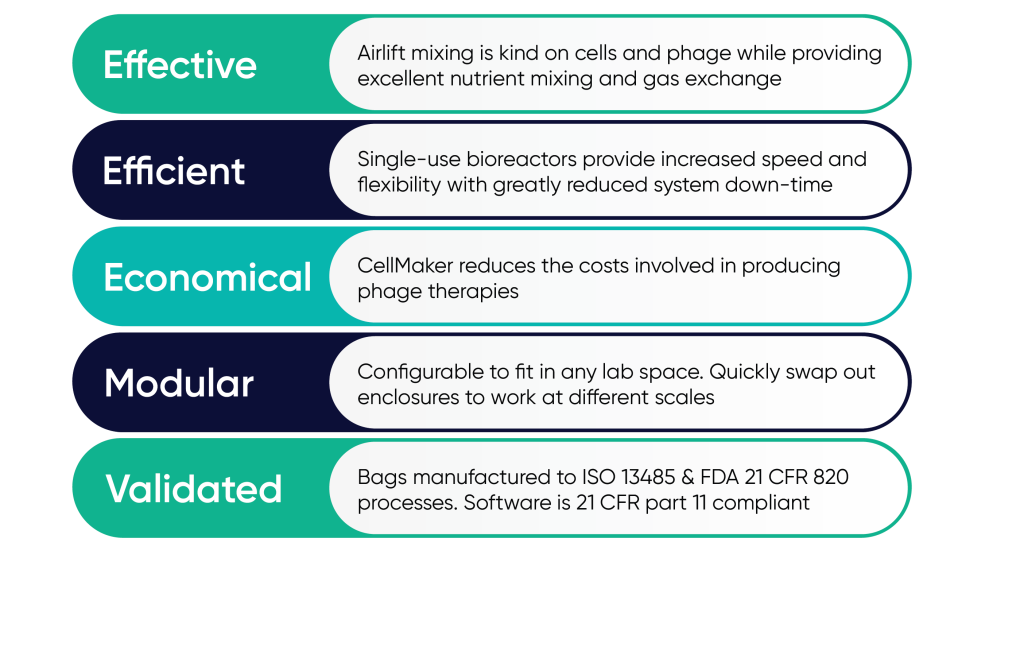
As the number of clinical trials for phage therapy increases, so does the demand for a reliable way to produce high titre phage particles. This requires a reliable culture platform that can readily switch between both research and therapeutic production to support this vital work. At the forefront of phage production technology is Cellexus. Their CellMaker platform can often produce phage at a titre of 10-100x higher than achieved using other bioreactors, this is due to its unique airlift mixing technology. Plus, its single use bioreactor bags eliminate cross-contamination issues and the need for time and resource consuming cleaning and sterilisation between runs. The CellMaker bioreactor is compact, modular and ready to use in a matter of minutes. Its software provides full control and logging of the culture process and can operate in research mode or in CFR Part11 compliant GMP manufacturing mode as required.
Surprisingly, phage therapy predates the use of antibiotics. In the 1900s, phage preparations were successfully used as a treatment for bacterial dysentery. However, they fell out of favour once penicillin, which was cheaper and easier to produce, became readily available. With current advancements in high-throughput laboratory techniques, safe and effective phage therapies could once again be the solution to the present-day problem of bacterial antibiotic-resistant infections.
(1) GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990-2021: a systematic analysis with forecasts to 2050. Lancet. 2024 Sep 28;404(10459):1199-1226. doi: 10.1016/S0140-6736(24)01867-1. Epub 2024 Sep 16. PMID: 39299261; PMCID: PMC11718157.
(2) Strathdee SA, Hatfull GF, Mutalik VK, Schooley RT. Phage therapy: From biological mechanisms to future directions. Cell. 2023 Jan 5;186(1):17-31. doi: 10.1016/j.cell.2022.11.017. PMID: 36608652; PMCID: PMC9827498.