White Paper: Amplification and transfection of CHO suspension cells in the CellMaker Low Flow bioreactor
Adam Ostrowski1, Scott Cameron2, Sean Brown2
1Cellexus International, Dundee, UK; 2University of Abertay Dundee, Dundee, UK
Background
The Chinese Hamster Ovary (CHO) cell line, and its derivatives, are the most commonly used eukaryotic cells in industrial biotechnology. These cells are comparatively easy to condition for growth in suspension culture, therefore many lines were permanently converted into suspension growth and are commercially available. These cells can produce large quantities of secreted protein, even 500mg of protein per litre of culture, with a degree of post-translational glycosylation. Another useful characteristic of the CHO cells is their high resistance to stress as well as relatively easy to meet trophic requirements.
The Cellexus CellMaker Low Flow single use bioreactor system (Fig. 1) has been specifically designed to accommodate amplification of mammalian cells, either in suspension or grown on a microcarrier support. The CellMaker range of bioreactors use the Airlift principle to move and mix the fermentation media and to deliver the gases required by the cells to grow. The performance of this innovative fermentation technology has been tested and proven superior in fermentation of bacteriophages, bacterial proteins and monoclonal antibody production from the Hybridoma cell line. However, the CHO cells have not been attempted to be maintained in this system previously. In this study, we embarked to verify that the CellMaker Low Flow system is suitable for maintenance and amplification of CHO cells as well as in situ transfection and production of recombinant monoclonal antibodies (mAbs).

Figure 1. The CellMaker Low Flow setup in the lab environment, including the Aber Instruments Futura sensor attached to the bioreactor bag via a side port.
Methods
We elected to use the commercial Expi-CHO expression system available from Thermo Scientific. The system comes complete with the Expi-CHO cell line permanently conditioned for suspension growth, Expi-CHO chemically defined and serum-free expression media, transfection reagents as well as positive control DNA vector encoding a recombinant rabbit IgG and Expi-CHO feed media. All cell maintenance, transfection and storage procedures were performed according to the manufacturer’s instructions.
The cells were routinely maintained in flat bottom, vented conical flasks in an orbital shaker incubator, set to 37°C and 8% CO2. The CellMaker Low Flow single use bioreactor from Cellexus was used to grow the cells at the scale of 3L in the CellMaker Plus single use bioreactor bag. The CellMaker bioreactor automatically controlled and maintained media pH and oxygenation levels. Media alkalisation was automatically controlled by the CellMaker by controlled delivery of CO2. Foaming was controlled by addition of 50-100ppm of Antifoam C emulsion (Sigma Aldrich). The cell density in the CellMaker bag was monitored using the Futura sensor kindly donated for the purpose of this study by Aber Instruments Ltd.
The 8L CellMaker bag was filled with 3L and the fermentation conditions were set to 37°C, pH 7 with automatic control using CO2 flow and air flow set to 0.5lpm. Once the Expi-CHO Expression medium reached the temperature setpoint, the bioreactor bag was seeded with 1.5×109 cells from a flask culture of CHO cells injected using a syringe via the inoculation port. The culture temperature was lowered to 32°C after transfection and feeding of the cells.
To isolate and concentrate the produced antibodies, cells were removed from the culture medium by centrifugation and filtration. 300ml of the culture supernatant was passed through an equilibrated 5ml Hi-Trap Protein A FPLC column (Cytiva) to trap the produced IgG and eluted with 15ml of 0.1M citric acid according to manufacturer’s instructions. The presence of the IgG was analysed by SDS-PAGE and Western blot using goat anti-Rabbit antibodies. IgG concentration in the elution fraction was quantified using Qbit.
The control experiment was conducted in a 125ml flat-bottom vented conical flask. CHO cells pre-grown to density of 107cpm were diluted to 6×106cpm in 30ml of fresh Expi-CHO media. The cells were transfected with the control plasmid according to the kit manufacturer’s conditions and the cells were incubated for 7 days at 37°C and 8% CO2 with horizontal agitation. The cells were fed with the Expi-CHO feed media one day after transfection. The culture supernatant was collected, purified using the 5ml Hi-Trap Protein A column and 15ml of 0.1M citric acid according to manufacturer’s instructions. The quality and quantity of the IgG recovered from the control culture was compared to the product from the CellMaker fermentation.
Results
Production of antibodies, or other proteins, in the CHO cell line at the scale of several litres requires large incubator capacity and multiple vessels. Due to space and equipment constraints, the maximum capacity of the laboratory, where this trial was performed, was approximately 500ml. We wished to increase the production scale to minimum of 4L and to this end we decided to use the CellMaker Low Flow single use airlift bioreactor system (Fig. 1). Prior to setting up the CellMaker for CHO cells culture for antibody production, we revived and amplified the cells using traditional culture in a flat bottom, vented conical flasks. The manufacturer of the protein expression kit suggests performing the transfection step when the cells achieve the density of 6×106 cells per ml (cpm) of media. Seeding this quantity of cells directly into the CellMaker Bag requires 18×109 of cells. Due to laboratory capacity constraints, we were unable to prepare such inoculum. Thus, we decided to grow the maximum quantity of cells in flasks – 1.8×109 of cells and continue the amplification in the CellMaker. We measured the cell density in the bioreactor bag continuously using the Futura capacitance sensor (Aber Instruments), which confirmed the initial inoculum at 5×105cpm. The airflow through the system at 0.5lpm was sufficient to maintain oxygenation of the cells and the automatic pH control system was periodically sparging the media with CO2 to reduce alkalisation and ensure CO2 is present in the media culture. After 48h the cells have reached the density required for transfection. The transfection reagent provided in the protein expression kit was mixed with purified plasmid DNA carrying the rabbit IgG gene to be expressed, and the transfection mixture was injected into the bioreactor bag. The cells were allowed to grow for another 24h. A small adjustment to the airflow was necessary at this point, as the increased biomass consumed more oxygen (Fig. 2b-d). After 24h, the culture was fed with the Expi-CHO feed medium, as instructed by the manufacturer. 500ml of media was injected using a peristaltic pump and the temperature was adjusted to 32°C. At this point, the Futura sensor recorded an expected drop in cell density, as the culture was diluted (Fig 2a, 70h). The culture continued as expected for another 5 days. At this point another 500ml of Expi-CHO media was added as a second feed. The Futura sensor once again has recorded the expected drop in the cell density in the culture media (Fig 2a, 160h).
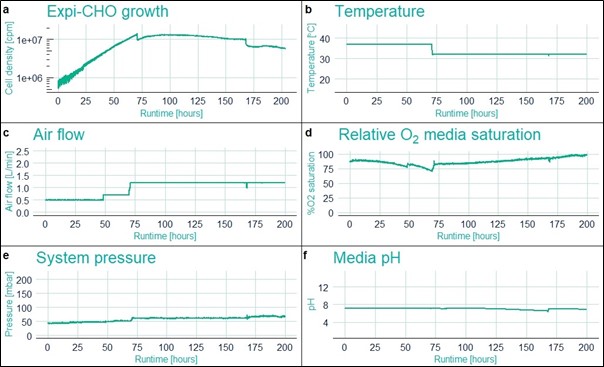
Figure 2. Fermentation parameters captured during the run by the Futura sensor (a. Cell density) and the CellMaker sensors (b-f).
Approximately 24h after second feeding, foaming in the bioreactor bag has increased substantially. This was most likely due to diluting out of the antifoam agent by the added feed media. At this point the cell culture was stopped and the media collected for analysis of IgG. The cells were removed by centrifugation, the supernatant filtered. To purify and concentrate the expected IgG present in the media supernatant, we passed the 300ml of the supernatant via a 5ml Hi-Trap ProteinA column and eluted the antibodies with citric acid onto Tris-HCl pH 9. The samples were analysed by SDS-PAGE for purity. The IgG elution was suspended in a loading buffer under reducing conditions and a series of 2-fold dilutions down to 1/64 was prepared. Although some additional bands are visible, the predominant bands visible in the gel (Fig. 3) correspond to the expected molecular weight of both heavy and light chains of a rabbit IgG (approx. 78kDa), as well as two bands, approx. 28 and 50kDa, suggesting incomplete dissociation of heavy and light chains. This is consistent with the expected sizes of the light and heavy chains of a rabbit IgG, respectively.
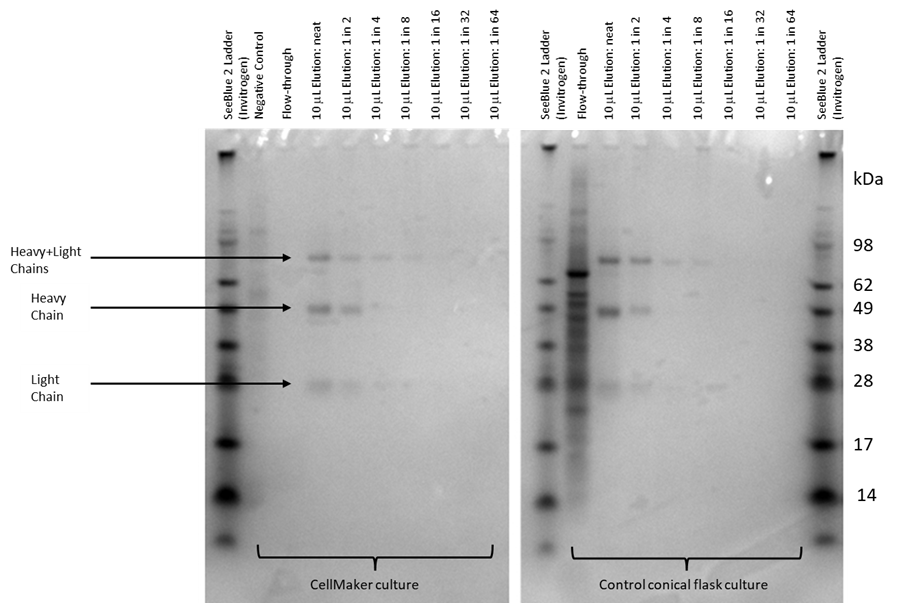
Figure 3. SDS-PAGE analysis of the IgG purification from the CellMaker fermentation and the control flask fermentation. Lanes showing the negative (not transfected) culture supernatant and the flowthrough the Protein A column are indicated. 10μl of sample in a 5x Leamlii loading buffer were loaded either undiluted (neat) or after serial 2x dilutions. The bands corresponding to the light, heavy and light and heavy chain complexes are indicated. The molecular size of the bands was estimated using the SeeBlue 2 protein standard.
The IgG recovered from the control flask culture have produced bands of a similar pattern seen in the samples from the CellMaker. Western blot analysis has confirmed the isolated protein as a rabbit IgG (Fig. 4). The bands corresponding to the light and heavy IgG chains, as well as a heavy and light chain complex, were the only products recognised by the secondary anti-Rabbit antibody. The recovered antibodies were quantified using the Qbit system for 18.8μg/ml and 411μg/ml from the CellMaker and flask cultures, respectively.
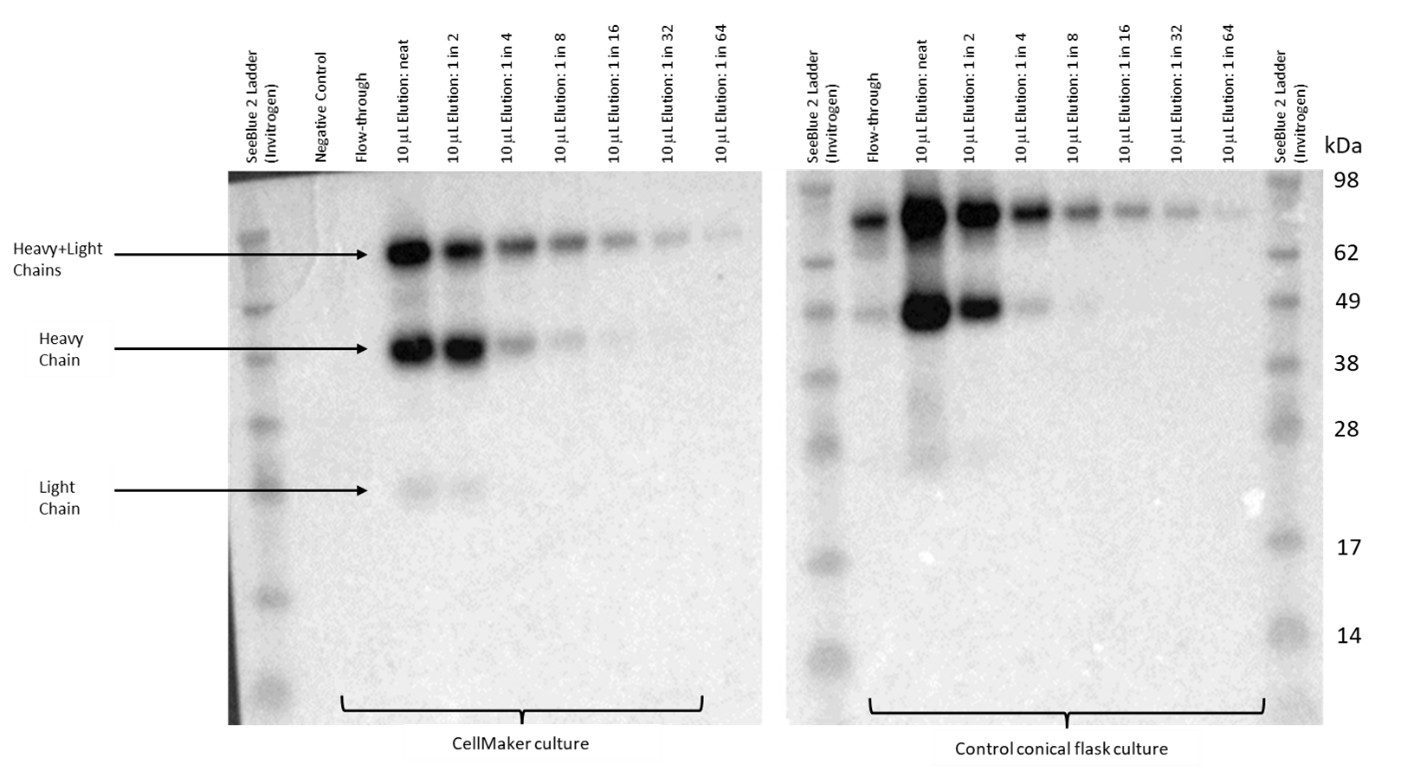
Figure 4. Western blot analysis of the CHO cell line culture supernatant for presence of recombinant rabbit IgG. Samples of negative (untransfected) control media supernatant, the flowthrough the Protein A column and the eluent containing concentrated antibody were loaded onto an SDS-PAGE gel and transferred to a PVDF membrane. The membrane was exposed to the secondary anti-rabbit HRP-conjugated antibody and visualised using an ECL reagent. The antibody eluent was serially diluted by factor of 2 for comparative analysis to the control cell culture in a conical flask. The bands corresponding to the light, heavy and light and heavy chain complexes are indicated. The molecular size of the bands was estimated using the SeeBlue 2 protein standard
Conclusions
In this proof-of-concept study report we have shown that the CellMaker Low Flow single use Airlift bioreactor system is a convenient instrument to upscale culture of suspension cells, like the CHO cell line, for production of recombinant proteins. The automated control of fermentation parameters, like media pH and aeration level, by the CellMaker ensured that the ideal growth conditions are maintained throughout the fermentation. Additionally, the on-line cell density monitoring using the Futura sensor allowed us to pinpoint the ideal moment for transfection. As the Futura sensor detects only the living cells, this allowed us to instantly ensure the cells in the culture remain viable without the need of taking samples and assessing the cells’ condition under a microscope.
In this example we used a recombinant protein consistent with the characteristics of a rabbit IgG which was successfully produced in the Expi-CHO expression system we used in this trial. The CellMaker enabled us to grow a cell culture at a significantly larger scale, than the traditional methods allow in the current setup of the lab. The maximum culture volume supported by our incubators is approx. 500ml, whereas in the CellMaker we could maintain a 3L initial culture with ease and with option of further scale-up to 8L in the hardware used in the experiments presented in this report. The identity of the proteins shown in the Figure 3 was confirmed using Western blot as rabbit IgG (Fig. 4). The purified samples were analysed by gel electrophoresis in a range of dilutions from undiluted to 1/64 dilution factor showing similar band patterns. The protein gel electrophoresis and Western blotting have confirmed the purity of the recovered antibody, which was also recognised by the anti-rabbit IgG secondary antibody. Quantification of the IgG recovered and purified from the CellMaker culture showed lower yield than from the control flask culture. The concentration of the IgG after purification from the CellMaker culture was 18.8μg/ml, whereas 411μg/ml solution was recovered from the control (Table 1). However, the control flask was incubated at a higher temperature due to technical limitations and further investigation is required to verify if pre-growing of cells in the CellMaker had an impact on transfection efficiency.
In this study we have demonstrated that CHO cells can be easily grown in the CellMaker Low Flow system to high densities (1.34×107cpm, Fig. 2a) at volumes significantly larger than in a traditional flask culture. The automated parameter monitoring and control provided by the CellMaker significantly reduced the labour required to maintain the culture and provided a digital log of the culture conditions. We have also successfully transfected the CHO cells within the CellMaker bag, which enabled seamless initiation of antibody expression. The variety of injection ports included in the CellMaker bag allowed us to add all necessary additives, namely anti-foam, transfection reagents, the media feed line for culture feeding post-transfection, and sodium hydroxide for pH balancing of the culture. The connectors can be easily maintained in aseptic state, protecting the culture from contaminations common in shaker flasks. The system automatically controlled the supply of carbon dioxide to maintain pH and provide CO2 required for the cell growth. pH monitoring was provided by the pH probe included with the system, and the additional probe ports allowed us to connect a cell density sensor to monitor cell growth without the need to sample the culture and enumerate the cells off-line. The final monoclonal antibody yield recovered from the CellMaker culture was lower than that in the control flask. However, there are many parameters, which could affect expression efficiency, including optimisation of transfection or extended culture time at reduced temperatures. Due to unexpected foam build-up, the experiment presented here run for a shorter period than initially planned. Further optimisation of culture conditions would be required to identify the best possible conditions. Nonetheless, we have proven successful antibody expression from CHO cells using a commercially available protein expression system in the CellMaker Low Flow bioreactor system.
| Culture volume | Purified antibody concentration | Total antibody produced | |
|---|---|---|---|
| CellMaker culture | 400ml | 18.8μg/ml | 75.2mg |
| Control flask culture | 30ml | 411μg/ml | 12.3mg |
Table 1. Quantitative comparison of monoclonal antibodies produced by the Expi-CHO cells in the CellMaker and flask cultures
Acknowledgements
This work is supported by IBioIC Feasibility Project grant (Project No FF-2021-04) to the University of Abertay Dundee. We thank Dr Rachel Crossley, Aber Instruments Ltd, for her continuing support regarding the Futura cell density sensor.