The Phage Maker: Making your bioreactor work for you
Introduction
With bacteriophage research moving at an exciting pace, the next stage is inevitably increasing your production volumes. Although scale-up isn’t always planned out at the beginning of developing bacteriophage products, it can play a paramount role in the feasibility stages and regulatory approval of the project. Whilst the production of phages in laboratories is considered a routine process with already established protocols, many of these methods do not transfer well to a scaled-up operation.
Bacteriophage production is a delicate balance of two processes: growth of host bacteria and infection of bacteriophage (propagation). Both require their own optimal conditions (e.g., temperature) and finding the correct operation procedure can be quite tricky. Furthermore, each phage has its own properties in culture (adsorption rate, burst size, latent period). Knowing the individualized biology of each bacteriophage and their respective infection parameters (Fig. 1) is vital to model its behavior and predict the optimal fermentation conditions.
Figure 1: Bacteriophages from Siphoviridae, Podoviridae and Myoviridae families with different morphology and biological properties. © Ninjatacoshell, images shared under Creative Commons Attribution-Share Alike 4.0 International license.
Computer simulations are an effective tool for bridging the elusive gap between shake flask and large-scale bacteriophage production. These have been done for multiple processing configurations (continuous, batch, two-stage). However, for many practitioners this is considerably further down the project timeline. For now, most wish to simply achieve trusted scale-up from flask. From conversations with scientists, high titers and ease of use is consistent on everyone’s wishlist. My hope for this blog is to give insight to the how the CellMaker has brought success to many of our users for both commercial and academic applications.
Airlift Technology
The CellMaker bioreactor system is complete with a patented enclosure and a single-use bioreactor bag which utilizes a unique airlift technology to move cells and nutrients: eliminating the need for mechanical stirring. The CellMaker technology is driven by sparging of air through the bioreactor, ensuring good aeration of the culture. The CellMaker PlusTM system monitors DO in the bioreactor bag in real time and automatically adds oxygen from an external source, if necessary. The CellMaker system can support third-party cell density monitoring equipment in the event where you wish to further optimize the MOI and infection point of your process, or simply monitor the progression of the culture. The CellMaker was designed with flexibility in mind, which means it can also support fermentation of phages against microaerobic or even anaerobic hosts. The adjustability of the inlet gas supply means the choice of inlet composition (N2, CO2, H2) can be tailored to your process. With the CellMaker built in Peltier heating unit, accurate temperatures can be easily controlled from 16°C to 40°C with the precision of ± 0.1°C.
As you are aware, the factors that influence phage amplification is extremely complex. With that being said, we believe the low shear environment the CellMaker provides generally make the cells very happy (happy cells = ample phages!) and the phages remain undamaged as there are no mechanical impellers inside the fermenter. Now for some real-life success stories!
Case Studies
Prof. Rob Lavigne’s laboratory at KU Leuven utilized the CellMaker to amplify phages against the plant pathogenic gram-negative Agrobacterium which causes infections in some plant species. With traditional treatment involving undesirable harsh chemicals, bacteriophages offer a viable alternative. In this study, KU Leuven compared phage amplification between traditional 500mL Erlenmeyer flasks to the CellMaker regular 8L system. As shown in Fig. 2 below, the CellMaker achieved a 12.5x greater final lysate titer to the traditional flask operation (despite inoculating at lower concentration of bacterial host).
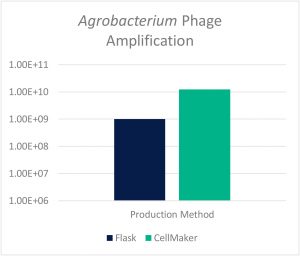
Figure 2: Final Lysate Titer Comparison: Shake Flask vs CellMaker Regular 8L System
Click here to read our application note for the above study.
For commercial scale, we are proud to be supporting the production efforts of Proteon Pharmaceuticals, who’s production line is based on the CellMaker Regular and Dual systems as seen below in Fig. 3.

Figure 3: Proteon Pharmaceuticals Fermentation Line
Proteon’s BAFASAL phage product has recently received a positive recommendation from the European Foods Standards Authority. Proteon cites the disposable technology of the system as a key advantage. More information on how Proteon has benefited from the CellMaker can be found here.
Single Use Technology
Cellexus believes in making a system that works for the user. It is often the case that increasing the number of system’s functionalities can complicate (sometimes even curb) its operation, and therefore, production. A feature that many of our user’s love is the ease-of-use due to the single-use system. With the CellMaker, every reactor bag starts the process afresh which ensures complete sterilty and gives an added level of confidence, especially when expensive culture medium is used. Furthermore, labs that produce a library of more than one phage can be assured there is crossover and therefore no unwanted phage ‘cocktails’ in their product. A perceived downside of SUBs is the environmental impact associated with plastic waste. My colleague Dr Adam Ostrowski has discussed this in another blog post, detailing how the reality of single use sustainability issues is surprisingly much more complex than what is viewed.
Conclusions
In summary, we at Cellexus believe strongly that a bioreactor should work for you and not vice-versa. With the CellMaker you can spend more of your time on the real science and let the CellMaker amplify your bacteriophages. Our system automates your processes, offering batch to batch reproducibility and volume efficiency with excellent titers. If you wish to learn more about how the CellMaker can help your scale-up endeavours, please feel free to contact myself or any of the Cellexus team. If you wish to read more about how the CellMaker has been successful in bacteriophage amplification, please view our application notes!
Author
 | Robert Matthew Technical Application Specialist at Cellexus Robert is a Technical Application Specialist at Cellexus where he helps customers find the optimal combination of bioreactor solutions & achieve the best possible results using our technology. Robert has a MEng in Chemical Engineering and a MSc in Industrial Biotechnology from Strathclyde University. |